Neuronal Morphology
Cell Bodies
Unlike the cells of any other organ in the body, each neuron has a unique morphology (Figure 1). This uniqueness in structure reflects their distinctive functions and underscores the immense power of computation in the brain. This uniqueness also makes it very difficult to study the nervous system, however, based on certain similarities in their structure such as cell body shape or size and dendritic or axonal distribution, it is possible to classify specific groups of neurons into categories. These neuronal categories or classes of cells can be studied in detail using electrophysiological techniques. Features of their structure provide clues concerning cellular function can be gleaned from this characteristic. For example, large irregular shaped or multipolar neurons often have long processes. These cells typify the neurons found in the motor pathways. Neurons with pyramidal cell bodies are quite common in the cerebral cortex where the cells and fibers are stacked in non-repeating layers. These elongated cells typically feature one or more processes that span several layers of the cortical structure. Globular to round shaped perikarya are typical of neurons found in brain stem sensory pathways. The processes of these cells usually are highly branched and densely packed together.
In general, the size of the neuron cell body gives some indication of the distribution of its axon. The large neurons tend to have long axons that project to other nuclei in the different regions of the brain or spinal cord or into peripheral nerves. These cells are often called projection neurons or Golgi type I neurons in honor of the famous Italian neurocytologist, Camillo Golgi (Figure 2). Neurons with small perikarya typically give rise to highly branched axons that remain close to the cell body of origin. Such cells are often referred to as local circuit neurons or Golgi type II cells. Although these generalizations concerning structure‑function correlations provide helpful insights, there are some exceptions to the rule.
The cytoplasmic membrane of each neuron in the brain is completely covered by either the small end feet of glial cells such as the astrocytes or by the presynaptic terminals of axons. No portion of the neuronal membrane is free from contact or exposed to the extracellular fluids of the central nervous system. The glial-neural contact serves a protective function, keeping the neuron isolated from the surrounding extracellular fluids, as well as a metabolic function, supplying the neuron with lactate derived from glucose. In addition glial cells remove waste metabolic products from neurons; thus the glial-neural relationship has been described as a metabolic coupling between these two cell types serving to maintain homeostatic conditions in the central nervous system (Tsacopoulos and Magistretti, 1996).
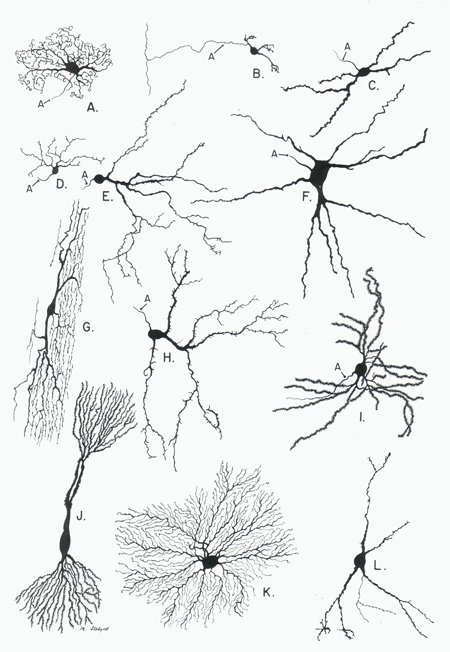
Figure 1 Examples of neuronal dendritic domains taken from spinal cord (G), brainstem (A,C,D,E,F,H), cerebellar cortex (B), thalamus (K), basal ganglia (I,L) and hippocampal formation (J). The extreme variations in dendritic domain shape reflects the varied function of these neuron. (Figure taken from Parent, A. Carpenter's Human Anatomy, Baltimore:Williams & Wilkins, 1996.)
The cytoplasm of the neuronal perikarya contains many features of typical mammalian cells, i.e. mitochondria, Golgi body, smooth and rough endoplasmic reticulum, free ribosomes, storage vacuoles, a cytoskeleton composed of microtubules (neurotubules) and microfilaments, peroxisomes containing oxidative enzymes and inclusion bodies containing metabolic waste products. Accumulations of rough endoplasmic reticulum are referred to as Nissl bodies because they stain intensely with cresyl violet, a stain commonly used in neuropathology laboratories (Figure 3). Alterations in the content and arrangement of these structures are the hallmark of numerous neuropathological processes.
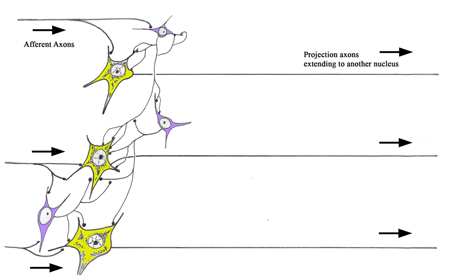
Figure 2 A diagram illustrating the difference between Golgi type I and II neurons. Golgi Type I neurons (yellow) have long axons that project to another nucleus in the brain. Golgi Type II neurons (blue) have short axons that remain within their local area. The Golgi Type II neurons have played a critical role in the development of the human brain. They account for the majority of the increased numbers of neurons present in the human brain when compared to those of the non‑human mammalian species. Their presence in a neuronal circuit allows for a much more sophisticated level of neuronal computation. Each Golgi type II neuron (interneuron) can interconnect with many projection neurons. Some of the interneurons are excitatory and some are inhibitory, thus these cells are capable of greatly altering the processing of information in a nucleus.
Dendrites
Almost all mammalian neurons have elongated cytoplasmic extensions referred to as dendrites (Figure 1). Since these processes represents extensions of the neuronal cell body they typically have the internal characteristics similar to the neuronal cytoplasm such as endoplasmic reticulum and mitochondria. However, unlike the cell body, the dendrites contain a highly organized cytoskeleton consisting of an array of oriented microtubules and filaments (Peters, Palay et al., 1991).
The total spatial distribution of the neuron's dendrites is referred to as its dendritic domain. As with perikaryal shape and size, there is enormous variation in the size and shape of individual dendritic domains. This is a very important feature of the neuron because the dendritic distribution, in part, determines the interaction between the neuron and its surrounding connections. Flatten dendritic domains can restricted input to a cell, focusing the neuron on a given activity, whereas round of bushy dendritic domains can allow the cell contact with many different inputs making it more of a universal responder. Given the elaborate complexity of dendroaxonic interactions it is no wonder that we are capable of complex behavioral patterns in our lives.
Dendritic arbors are often depicted as static structures, formed during neurogenesis and maintained throughout life. However, studies have suggested that dendrites are very plastic and change shape and position with time. This has been demonstrated by observing over a three month time period, the dendrites of neurons in the superior cervical ganglion stained with a vital dye (Purves, Hadley et al., 1986; Purves, Voyvodic et al., 1987). During this period, some of these dendrites retracted back towards the cell body, some elongated and others arouse de novo from the ganglionic neurons. Constant changes in dendritic domains may underlie some of the processes occurring in learning and memory. Conversely, atrophic changes in dendritic structure can occur in response to stress such as the prolonged exposure to elevated levels of glucocorticoids during stressful events of either a physical or psychosocial nature (McEwen, 1999).
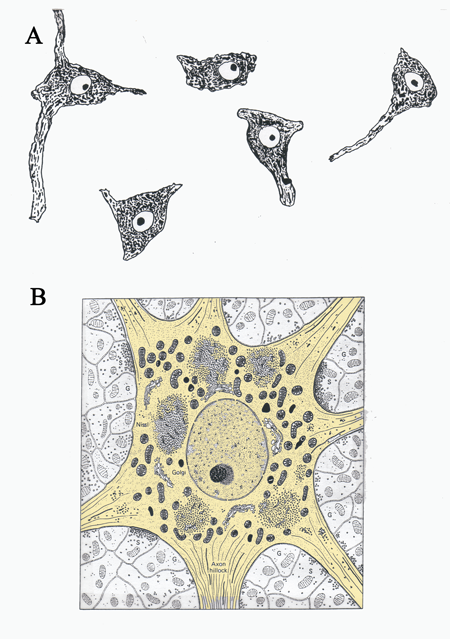
Figure 3 A.) A drawing of Nissl stained neurons seen under a light microscope and B.) a drawing of an electron micrograph of a neuron. The cell body of the neuron is shaded light brown. (Illustration in B taken from Junqueira, L.C., Carneiro, J., And Kelly, R.O., Basic Histology, Appleton and Lange, Norwalk, CT,)
Dendrites function to increase the membrane surface area of the cell and to allow the cell to receive information from a variety of sources. The dendritic arbor and the perikarya act as the input surface for the neuron, most of the cell's synaptic contacts occur through these two structures. The output from a neuron occurs through its axon. This relationship creates a one‑way flow of information through a neuronal circuit and has been termed the law of dynamic polarization. Specializations such as small spines are present on many dendrites in the central nervous system. These spines function to increase the surface area of the dendrite and play a role in modulating the movement of ions into the cell (Segal, 1995; Shepherd, 1996; Hering and Sheng, 2001).
In general, motor neurons tend to have long dendrites that radiate away from the perikarya, such as the ventral horn motoneurons of the spinal cord; conversely, sensory neurons in the CNS tend to have shorter, but highly branched dendrites that often exhibit a planar distribution. One of the most distinctive neuron types in the mammalian CNS is the pyramidal cell found in the cerebral cortex and hippocampal formation (Figure 1 cell j). The morphology of this cell is a critical determinant of its function. The long apical dendrites of the pyramidal cells are arranged vertically across the layers of the cerebral cortex. Many different synaptic inputs end on these dendrites as they pass through each layer of the cortex. The pyramidal cell is capable of integrating information from multiple layers of the cerebral cortex. The common orientation of these dendrites from millions of pyramidal cells allows the extracellular recording of a combined electrical potential termed the electroencephalogram.
Axon
Neurons have two fundamentally different types of processes: dendrites that conduct information toward the cell body and axons that conduct information away from the dendritic or receptor portion of the neuron (Peters, 1968). The differing functional roles for these processes are reflected in their differing morphology.
The axon is the major vehicle through which the neuron expresses itself. Typically axons arise abruptly from the cell body or a proximal dendrite. Two major components of the axon are described: 1) the axon proper and 2) the surrounding myelin sheath (Figure 4). The axon proper is a thin column of cytoplasm, surrounded by a membrane and containing no rough endoplasmic reticulum or Golgi apparatus. It does, however, contain smooth endoplasmic reticulum, mitochondria and is packed with neurofilaments and microtubules oriented along the long axis of the axon. Unlike the cytoplasm of the cell body and dendrites these fibrous elements are phosphorylated (Hirano and Llena, 1995). The axon proper is surrounded by a cytoplasmic membrane, termed the axolemma, outside of which lies the myelin sheath. The myelin sheath consists of the cytoplasm and associated membranes of surrounding glial or Schwann cells wrapped around the axon proper (Figure 4). Importantly, the number and thickness of the wraps varies increases with the increasing conduction velocity of the axon.
The distribution of the axon directly affects the ability of the parent neuron to process information. Two general classes of neurons are recognized based on axonal distribution. Golgi type I cells typically have long axons that extend into other regions of the CNS. The axons of the Golgi type I cells bundle together in the brain and spinal cord to form structures termed fiber tracts or pathways (Figure 2). Golgi type II cells have short, highly branched axons (axon branches are called collaterals) that innervate the region surrounding the cell body of origin (Figure 2). These latter neurons are sometimes referred to as local circuit neurons or interneurons, and their function is to moderate the activity of surrounding Golgi type I neurons.
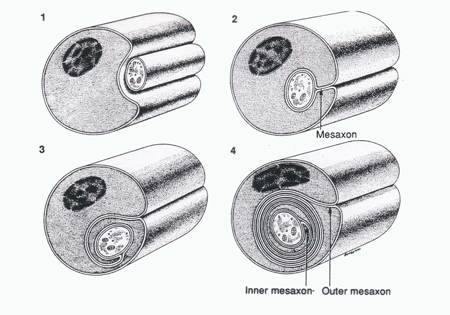
Figure 4 A diagram illustrating the relationship between Schwann cell (outer covering) and the axon cylinder. (Taken from Junqueira, L.C., Carneiro, J., And Kelly, R.O., Basic Histology, Appleton and Lange, Norwalk, CT, 1989).
The distribution or domain of the axon determines the influence of the parent neuron on the target population. A restricted axonal distribution allows the parent cell to effect only a limited number of target neurons, this type of arrangement will preserve the fidelity of the signal and is termed convergent and is typical of many Golgi type I cells found in sensory pathways. A broad distribution of the axon allows the parent cell to cell communicate with many target cells thus widely distributing its signal within the nervous system. This type of organization is termed divergent and is typical of Golgi type II neurons or interneurons found within the nuclei of sensory pathways. Both types of axonal arbors – convergent and divergent – are present in the mammalian brain.
One should not think of the connectivity of cells in brain tissue as being static. Evidence suggests that the axonal arbors in parts of the cerebral cortex, similar to dendrites, are in a state of constant dynamic flux, remodeling and rearranging their distribution as the individual processes information (Antonini and Stryker, 1993). This dynamic state of the axon is also considered to be one of the morphological correlates of learning and memory.
All axons are maintained within protective coverings formed by the membranes of neuroglial cells. Some of these coverings consist of a single wrapping of the glial cell membrane; such axons are thus termed unmyelinated fibers. Other, more elaborate, coverings consist of multiple wrappings of the glial cell membrane (Figure 5). In addition, the glial cell membrane has become specialized for the production of myelin basic protein, a good insulating substance. Axons wrapped in this manner are called myelinated fibers.
Myelinated axons in the CNS are surrounded by oligodendroglial cell membrane (Figure 5); whereas those in the peripheral nervous system are surrounded by membranes of Schwann cells. In either case, a short, unmyelinated portion of the axon separates the wrappings of individual glial (or Schwann) cells. This gap is called the node (of Ranvier) and the remaining, myelinated portion is called the internodal segment.
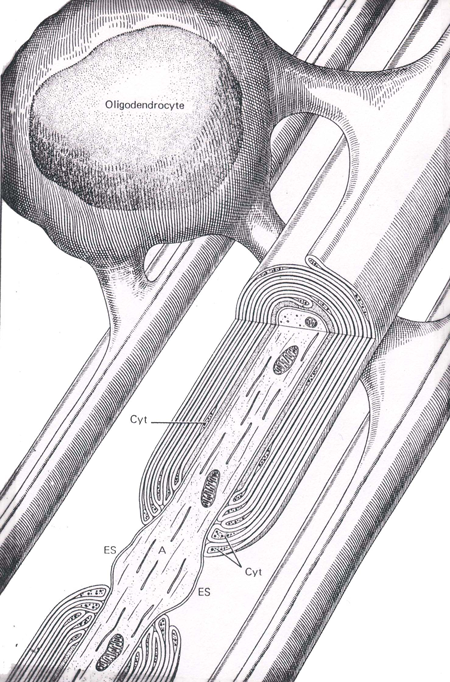
Figure 5 An illustration of an oligodendrocyte wrapping around several axons in the central nervous system (Abb.: A, axon; Cyt, glial cell cytoplasm; ES, extracellular space; IM, inner mesaxon; Taken from Junqueira, L.C., Carneiro, J., And Kelly, R.O., Basic Histology, Appleton and Lange, Norwalk, CT, 1989).
Oligodendroglial cells in the CNS, and Schwann cells in the peripheral nervous system, can form either myelinated or unmyelinated coverings for axons. Whether they produce myelinated or not, oligodendroglial cells can surround numerous axons; whereas Schwann cells, when unmyelinated surround multiple axons, but when myelinated surround only one axon.
Myelination of an axon greatly increases its conduction velocity. Instead of carrying the action potential along the entire length of the axon, it jumps (saltatory conduction) over the internodal segments, depolarizing the axon only at the nodes. Large myelinated fibers with their extremely fast conduction velocities serve many of the discriminatory and proprioceptive (position) sensory modalities as well as fine motor functions of mammals. These functions are the first to suffer in demyelinating diseases that result in the destruction of myelin around the large caliber axons. Small lightly myelinated and unmyelinated fibers with their slow conduction velocities are found in the pain pathways and are the predominant fiber in the pathways mediating peripheral autonomic functions.
The rapid saltatory conduction of myelinated nerve fibers is contingent on the segregation of specialized ion channel domains along the membrane of the axon (Waxman, 1982; Waxman and Ritchie, 1985). Membrane domains rich in sodium channels are sequestered in the nodes where they are exposed to the surrounding extracellular fluids while membrane domains rich in potassium channels are found covered by the myelin wraps (Figure 6). The high density of exposed sodium channels renders the nodal area quick to depolarize whereas the high density of covered potassium channels acts to block to movement of the depolarization along the axon. Demyelination, such as occurs in multiple sclerosis, results in the retraction of the Schwann cell wraps and exposure of the K+ channels. The inward current generated by exposed K+ channels acts to block the saltatory spread of current along the axon; thus shorting out the action potential.
Damage to an axon leads to degeneration of the section distal to the injury. This processes is called Wallerian degeneration and is heralded by disintegration of the axon followed by a similar demise of the myelin sheath. In the peripheral nervous system, Schwann cells, distal to the damaged section, undergo a hypertrophy and engage in phagocytosis of the axon remnants as well as the autodigestion of the myelin wraps. These same cells elongate and form small tubes through which the regenerating axon grows. Since Schwann cells are not found in the CNS, little axonal regeneration can occur. In fact, it is thought that the astrocytes of the CNS actually hinder the progress of axon growth in mature tissue. Studies using neurotrophic growth factors and antibodies to molecules such as the proinflammatory cytokines offer promise in finding a way to enhance regeneration and regrowth of axons in the central nervous tissue (Fawcett and Geller, 1998; Lu and Waite, 1999).
During Wallerian degeneration in the distal stump of the axon, changes are also taking place in the neuron soma as well. The endoplasmic reticulum disintegrates and the cytoplasm fills with vacuoles. Since these two events diminish the ability of the cell to react with Nissl stain, the overall process is called chromatolysis by the neuropathologist. The occurrence of chomatolysis following a lesion was used by many of the early neuropathologists to study connections in the central nervous system.
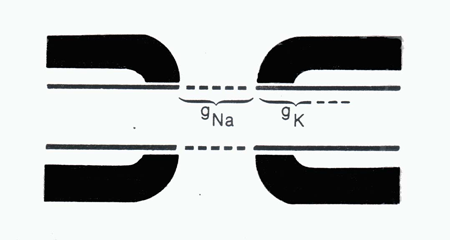
Figure 6 An illustation of an axonal node demonstrating the location of sodium (Na+ Ch) and potassium (K+ Ch) channels. The thick black lines represent myelin wraps. (Taken fromWaxman, S.G. Normal and abnormal axonal properties. In: Diseases of the Nervous System: Clinical Neurobiology, edited by Asbury, A.K., McKhann, G.M., and McDonald, W.I.Philadelphia:W.B. Saunders, 1986,p. 36-56.
Myelin is associated with several different proteins, glycoproteins, and phospholipids. As the myelin sheath degenerates in demyelinating diseases, such as multiple sclerosis and viral-induced encephalopathies, these chemical substances become detectable in the cerebrospinal fluid. Myelin basic protein and myelin-associated phospholipid proteins are the two major classes of proteins composing myelin in the central nervous system (Campagnoni, 1988). A minor myelin protein is called myelin-associated glycoprotein. When a demyelinating disease is suspected, examination of the cerebrospinal fluid by electrophoresis is performed; thick bands of homogenous protein, termed oligoclonal bands, in the electrophoresis gel are the hallmark of elevated myelin proteins levels. A common demyelinating disease of the central nervous system is multiple sclerosis.
Electrodiagnosis Peripheral nerves can be stimulated with an electrode and the resulting volley of action potentials recorded through a surface electrode implanted in the skin. Several parameters of the action potentials can be measured such as latency, duration, and amplitude. If the current used to stimulate the nerve is very low, only the large myelinated fibers will be activated. Their uniform size contributes to a fairly consistent conduction front along the nerve. Therefore a waveform can be recorded with an extracellular electrode placed at a distance, for example, on the skin overlying the nerve. Many disease processes affect these values and, therefore, can be diagnosed using electrophysiological methods. Examples of such pathologies are metabolic diseases (diabetes), post traumatic lesions, and genetic diseases.
Demyelinating Diseases A number of neuropathological disease processes feature the destruction of axon myelin sheaths. These demyelinating diseases initially present clinically as distortions in sensory system function, termed paresthesia, or as poor muscle coordination and culminate in the eventual loss of sensory and motor functions. Demyelination of a single peripheral nerve is referred to as mononeuropathy, a symmetrical (bilateral) disorder of the peripheral nervous system is called polyneuropathy. The causes of demyelination are many and varied. They can be inherited, acquired through environmental toxins, or can accompany other systemic diseases such as diabetes or viral infections.
An index of demyelination in a patient is the appearance of myelin break down products in the cerebrospinal fluid (CSF). Using electrophoresis of CSF, one can demonstrate the presence of myelin basic protein} (MBP) in patients with demyelinating diseases such as multiple sclerosis, neurosyphilis and subacute sclerosing panencephalitis.
Cytoskeleton
The cytoplasm of a neuron is contained within its plasma membrane. Within the cytoplasm there is a definitive order to the arrangement organelles. This order is established by the cytoskeleton of the cell which is made up of various types of filaments and microtubules. The cytoskeleton acts to anchor the cellular components in position and to give structural support to the overall form of the cell. If the cytoplasm of the neuron is removed, and only the cytoskeletal components remain, the shape of the cell will still be maintained (Hall, 1992).
The cytoskeletal elements of the neuron come in several sizes of diameter: microfilaments = 5 nanometers; intermediate filaments = 10 nanometers; microtubules = 20‑40 nanometers. Each type has a different distribution and function in the neurons.
Microfilaments These are the smallest of the cytostructural elements and are found in axon growth cones; they are made up of actin polymers. Microfilaments are largely responsible for movement of the cell processes.
Intermediate filaments These elements, also called "neurofilaments" are made up of fibrous proteins. They are least abundant in the nucleus, more so in dendrites and most abundant in axons. These filaments provide bulk and are critical to the strength and integrity of the cell. In certain pathological conditions, such as Alzheimer's disease, the neurofilaments accumulate in a disorganized mass to form a neurofibrillary tangle.
Microtubules These tubular structures are assembled from dimers of alpha‑ and beta‑tubulin, a protein. Since microtubules can assemble and disassemble rapidly they should be considered a very dynamic element in the cytoskeleton. Microtubules are involved in the extension of neuron processes and play a key role in the transport of material throughout the cell, especially with axon‑transport. Other structural and function protein or enzymes can attach to microtubules in the cell.
Neuronal Cytoskeleton and Disease
Numerous cellular proteins are involved in the regulation of growth and metabolism within neurons. Abnormal interactions between these proteins can determine the difference between homeostatic regulation and disease. PS-1 and PS-2 are newly discovered transmembrane proteins associated with the endoplasmic reticulum (ER) where they appear to influence vesicle and cell surface protein trafficking as well as ER calcium regulation. These two proteins are produced by presenilin alleles segregating at two loci, one on chromosome 14 (PS-1) and one on chromosome 1 (PS-2) (18). PS-1 and PS-2 protein also associate with components of the cytoskeleton; in this position they appear to influence the expression of another cellular protein, $-amyloid precursor protein (b-APP). The b-APP protein is a transmembrane protein involved in promotion of cell survival, stimulation of neurite outgrowth, stimulation of synaptogenesis, modulation of synaptic plasticity, regulation of cell adhesion, protection against a range of metabolic, excitotoxic and oxidative insults (19). The presenilin proteins appear to be involved in associating b-APP with the ER (20). A mutant form of b-APP , termed Ab-42, is heavily expressed in senile plaques; interestingly, mutations in either of the PS-1 and PS-2 alleles result in an increased expression of Ab-42 in neurons. Thus, mutation in the presenilin alleles may be the trigger that begins the deposition of Ab-42 amyloid plaque in neurons thereby precipitating the Alzheimer’s form of senile dementia (20). The abnormal deposition of protein complexes in tissue is a characteristic feature of numerous diseases such as Alzheimer’s disease, sarcoidosis, and amyloidosis. In reference to this mechanism of action, these pathological processes have been termed deposition diseases.
Synapses
The exchange of neurotransmitter between specialized regions of two cells is termed synaptic transmission. The synapse is the cytological device through which neurons communicate with each other. Due to their morphological and physiological properties, synapses essentially form one‑way channels or gates allowing the flow of information to proceed from the presynaptic to the postsynaptic membrane. Thus, synapses serve to rectify the flow of information in a neural circuit. The presynaptic component, located on an axon, is a bulbous ending, or terminal, containing a vesicular grid and synaptic vesicles with enclosed neurotransmitters (Figure 7). It is separated from the postsynaptic component by the synaptic cleft. The postsynaptic component contains a synaptic density. The membrane surrounding this structure contains the receptor molecules for specific neurotransmitters. In many neurons the postsynaptic surface is located on a small extension of the soma or dendrite called a spine.
The classical structure of a synapse is seen in the neuromuscular junction (Martin, 1992). The synaptic vesicles of this junction contain acetylcholine that is released into a narrow intracellular cleft to activate receptors on the postsynaptic membrane of the muscle (Figure 7). In the central nervous system, synaptic terminals containing glutamate or GABA resemble the neuromuscular junction in their precision of action. Other central nervous system synaptic terminals containing such substances as catecholamines, indolamines or the neuropeptides work on a slightly different mode of operation. The transmitter (or sometimes called modulator) can be released into the extracellular fluid where it diffuses among the surrounding neurons. This form of chemical communication has been called volume transmission (Agnati, Bjelke et al., 1992) and is an important mechanism in the overall regulation of neural activity in the brain. It is a major mechanism of neural communication in the hypothalamus and is important in the maintenance of homeostatic regulation by the autonomic nervous system.
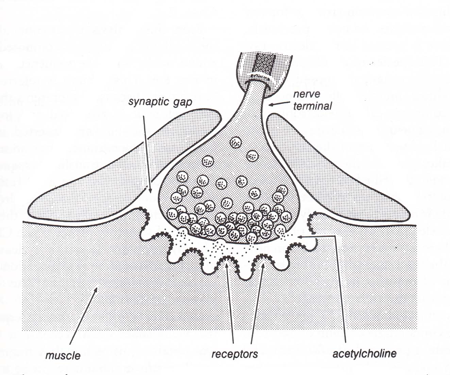
Figure 7 Diagram of a neuromuscluar junction. (Modified from Shephed, G.M., Neurobiology, Oxford University Press, New York, 1994.)
Synapses in the central and peripheral nervous system are not static. This cellular connections have proven to be very dynamic, being capable of expansion and contraction of size as well as increasing or decreasing their degree of security in connection. These changes have been termed synaptic plasticity (see textbox below) and underlie the process of memory and learning as well as some of the pathological changes seen in the development of chronic pain states.
Plasticity
Synapses are not static structures, often changing their properties based on the frequency or intensity of their activation. These activity-dependent changes are termed “plasticity” and provide a dynamic aspect to brain function. Various cellular methods of synaptic plasticity are known. One of which involves the opening of normally blocked channels in the synaptic cleft. These channels are attached to glutamate receptors but are blocked by a large divalent cation such as magnesium. Under intense activity, the cation is ejected from the channel and the channel is opened. In the opened or active state, the channel allows calcium ions to enter the cell which can activate enzymes and induce gene expression, thus altering (usually sensitizing) the activity of the cell. This mechanism of sensitizing a cell is present in the dorsal horn of the spinal cord and is thought to underlie one aspect of neuropathic pain (Dubner and Ruda, 1992).
Recently, it has been noted that the susceptibility of the neuron to plastic change can itself vary. That is, there are times when the neuron is more susceptible to plastic changes than others. This alteration in plasticity is termed “metaplasticity” and appears to be related to the activity of the calcium-dependent enzymes in the cell. Metaplasticity has been discovered in the hippocampal formation of the forebrain where it is involved in the process of memory formation (Abraham and Tate, 1997; Tompa and Friedrich, 1998).
The Ubiquitin-Proteasome Proteolytic Pathway
Protein synthesis, which is vital for neuronal survival, occurs in the endoplasmic reticulum of the cell. Neurons have to maintain long dendritic and axonal process that require both structural and enzymatic proteins. This demand is met through the activity of the endoplasmic reticulum (ER) and Golgi body (GB) complex located in the neuronal cell body. It is in the ER that the appropriate linear sequence of amino acids are united to form a long thin molecule. In the GB other modifications such as glycosylation, the adding of sugar moieties, is accomplished.
Proteins have a linear structure and a folded, three dimensional structure. Before passing from ER to GB, the linear string of amino acids has to be folded into a three-dimensional structure. It is this complex folded entity that is functional, not the linear string of amino acids themselves. Chaperone proteins help orchestrate the folding of the protein. These chaperones are located in the ER and not only assist in folding the protein by mark it for its final destination inside the cell. Secretory proteins will be marked for packaging into secretory granules, whereas structural proteins could, for example, be marked for transport by the microtubular system to the axon or dendrite.
Miss-folded protein have to be disposed by the cell (Kuznetsov and Nigam, 1998). Folding of proteins is a complex process and mistakes occur. When this happens, the miss-shapened protein is detected by the chaperones (and other systems as well) and marked for destruction. One aspect of this marking process involves the labeling of the dysfunctional protein with a ubiquitin protein. This marks the dysfunctional protein for inclusion into the proteasomes, membrane-bound vesicles that contain an assortment of proteolytic enzymes capable of breaking down the miss-folded protein and returning its component amino acids for reuse.
Defective disposal of miss-folded proteins leads to deposition of the protein about the cells and appears to the basis for several degenerative neuronal pathologies. The ubiquitin-proteasome proteolytic pathway can be overwhelmed with dysfunctional protein or there can occur mislabeling of protein such that it accumulates in the pathway with out degradation. In either event deposition of unusable protein occurs in the cell eventually leading to the death of the cell. This form of “deposition disease” appears to the basis several major diseases in the nervous system as well as in other organ systems. Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis are a few examples (Aridor and Balch, 1999; Glickman and Ciechanover, 2002).
Apoptosis: Programmed Neuronal Death
Neurons are born in very large numbers during development. This process is termed neurogenesis. Subsequently those that do not make adequate or appropriate synaptic connections during early development undergo a programmed cell death, termed apoptosis. In this process, a cascade of protease enzymes, the caspases, are unleashed eventually contribute to the demise of the cell. In essence, in apoptosis the cell undergoes a self autolysis. By dissolving itself gracefully, the cell does not trigger a massive inflammatory response such as would accompany the second form of cell loss, a necrotic cell death. This latter process of cell loss is typically seen accompanying traumatic cell injury and can evoke massive inflammatory responses that actually enhance the surrounding damage.
Apoptotic cell death is also occurring in mature individuals in association with oxidative stress (for a review see Friedlander et al, Friedlander, 2003). Neurons generate energy by oxidizing glucose to carbon dioxide and water in the tricarboxilic acid (TCA) cycle, also termed Krebs cycle. The high energy containing compounds generated in this cycle can be are very volatile and as such, can not exist for long in the cell, thus they are quickly used in the mitochondria to generate adenosine triphosphate or ATP. This later compound is less volatile and can be moved through the cytoplasm for use in other parts of the cell. The entire sequence of events is termed oxidative phosphorylation and is ubiquitous throughout the cells of our bodies.
The more demand placed on a neurons for high speed functioning, the more demand it places on it’s oxidative phosphorylation system. Even during normal functioning, this system of high-energy transfer between molecules sufferers from mistakes, such as the untoward release of free radical oxygen species. Loose free radicals are very volatile and quickly attach to other structures in the cell such as the cell membrane. This attachment process is damaging, creating holes in the membrane, and if done to a great enough intensity can trigger caspase enzyme cascades ultimately resulting in an apoptotic cell death. This process has been termed oxidative stress and forms the basis for numerous neuronal degenerative process.
Not all individuals suffer equally from oxidative stress. Neurons, as do other cells in the body, contain several protective systems to catch and disarm loose free radicals. The glutathione system and the superoxide dismutase enzymes are examples of free radical disarming mechanisms. Genetic variation in these systems can account for some of the different between individuals in their tolerance for free radical activity. Also, environmentally-mediated damage, such as toxins, to the either of these protective systems can enhance the effects of free radicals, thus accelerating neuronal degeneration.
