Neuroglia Morphology
The non‑neuronal, cellular elements of the central nervous system (CNS) are referred to as neuroglia; they represent supportive and protective components. The term neuroglia means "nerve glue" and as such these cells are packed very tightly around each neuron and its processes. This arrangement leaves little extracellular space in the CNS and effectively isolating neurons from each other as well as from the surrounding vascular system. They also form a limiting membrane on the external (pial) and ventricular surfaces protecting neurons from contact with cerebrospinal fluid. This latter function of the neuroglia is referred to as the blood‑brain barrier. Thus, neuroglia in the CNS are similar in function to Schwann cells and satellite cells of the peripheral nervous system. Beyond their role in support and the blood-brain barrier, neuroglia are also involved in development, metabolic homeostasis, immune surveillance and repair of the nervous system.
Development: Neuroglia play an important role in the development of the nervous system. Although the distinctive glial cell types are not present in the early developmental stages, their precursor, the radial glial fibers, form a complex matrix into which neurons migrate and eventually settle (Komuro and Rakic, 1995; Rakic, 1995). Principle among the many functions of these glial precursors are guidance of migrating neuroblasts, guidance of growing axons, maintenance of order in fascicles of axons, and providing trophic substances to sustain surrounding neurons (Noble, 1986).
Metabolic homeostasis: By isolating neurons from the body fluids, neuroglia can control what substances reach neurons and what metabolic by-products are removed form neurons. This arrangement has been termed “metabolic compartmentalization”. Breakdowns in the glial compartment can result in the metabolic poisoning of neurons.
Injury and repair: Unlike neurons, neuroglia retain the ability to multiply throughout life, consequently, they play an important role in the repair process following traumatic injury or infarction. A frequent result of tissue damage in the nervous system is the formation of a glial scar that involves excessive proliferation of glial cells. In fact some researchers feel that the lack of regeneration following injury to the mammalian CNS is due to the massive proliferation of glial cells. It appears possible that messenger molecules (cytokines), many of which are produced by glial cells during their post‑traumatic proliferation also act to block the regrowth of damaged neuronal fibers (Liuzzi and Lasek, 1987).
Inflammation: Glial cells can produce messenger molecules that interact with immunocytes, smooth muscle cells, and vascular endothelial cells. For example, interleukin‑1 a cytokine produced by inflammatory cells such as leukocytes, is also produced by astrocytes. Not only does this cytokine act on leukocytes and vascular endothelial cells, but it also acts in an autocrine manner to function as a growth stimulant for astrocytes (Giulian, Woodward et al., 1988). The production of such cytokines as the interleukins, interferons, and tumor necrosis factor by glial cells has been suggested as playing a critical role in the inflammatory response of the central nervous system (Merrill and Benveniste, 1996; Selmaj, 1992). Cytokines can induce all forms of glial cells to generate the matrix-metalloproteinases, powerful proteinases that can digest the surround extracellular matrix. The production of these proteinases is up-regulated in inflammatory diseases such as multiple sclerosis and in degenerative diseases such as Alzheimer’s disease (Yong, Krekoski et al., 1998).
Neuroglia can cause other serious problems in the central nervous system. Tissue damage in the CNS is often accompanied by disastrous swelling, resulting from the retention of fluid by glial cells. Finally, glial cells, being capable of cell division, are responsible for most CNS tumors. After injury to the brain, glial cells can proliferate wildly creating a massive glial scar which can block any possible regrowth of neuronal tissue.
Finally, several types of glial cells have been identified in the mammalian central nervous system. They differ in their morphology, function, and distribution (Figure 1). Each type will be briefly examined.
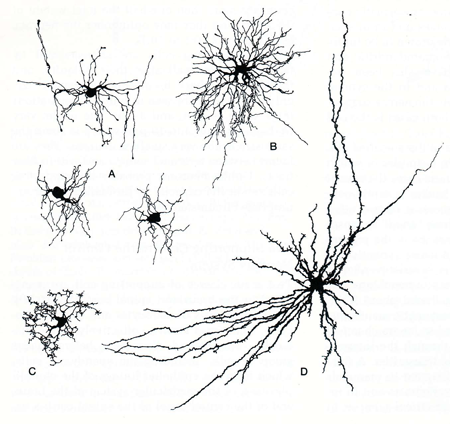
Figure 1. Examples of several types of neuroglia. A. Oligodendrocyte; B. Protoplasmic astrocyte; C. Microglial cell; D. Fibrous astrocyte. (Figure taken from Jones, E.G. and Cowan, W.M. The nervous tissue. In: Histology: Cell and Tissue Biology, edited by Weiss, L.New York:Elsevier Biomedical, 1977,p. 282-370.
Astrocytes are the most common type of glial cell in the central nervous system. These cells have many processes that radiate away from the cell body giving the appearance of a star. The processes form small end feet that attach on the exposed surface of neurons, surround blood vessels, and the inner surface of the pia and ependyma (Figure 2). Under the pial surface astrocytic end feet coalesce to form the external limiting membrane. Those that coalesce under the ependymal cells form the internal limiting membrane of the central nervous system. It is suggested that they have a transport function, moving selected substances across the blood‑brain barrier; indeed, for some substances these cells are believed to constitute the blood‑brain barrier. Astrocytes are interconnected through gap junctions to form a massive syncytium that can act as a storage depot for glycogen, amino acids and extracellular ions.
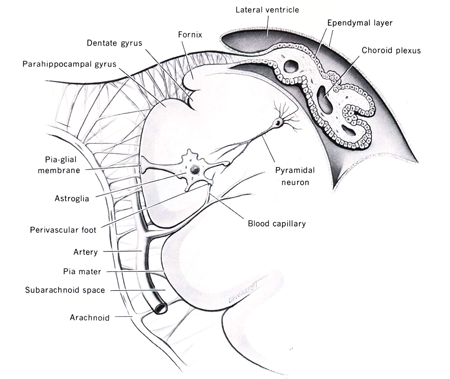
Figure 2: Diagram of astrocytes and their appendages in the central nervous system. The relationship between the choroid plexus and the ventricles is also illustrated. (Diagram taken from Noback, C.R. and Demarest, R.J., The Human Nervous System: Basic Principles of Neurobiology, McGraw‑Hill Book Comp., New York, 1981.)
Enlarge image in new window
Classically, two types of astroglia have been identified: fibrous and protoplasmic astrocytes. However, many transition forms exist; thus the two types probably represent the extremes of a morphological continuum. Fibrous astrocytes have long, thin processes that pack between blood vessels and neuron processes. Although they can be found in most parts of the CNS, they predominate in the white matter. Protoplasmic astrocytes are characterized by short, stubby processes that interdigitate between neuronal soma and dendrites. They are most commonly found in the gray matter.
Astrocytes have many functions beyond that of forming the “glue substance of neurons”. They play a major role in homeostatic balance in the central nervous system. Astrocytes function as a buffer for potassium ion balance by absorbing excess potassium ion and distributing it through out their syncytium. Evidence suggests that the release of potassium from astrocyte endfeet plays a role in regulating regional cerebral blood flow (Paulson and Newman, 1987). An equally important role for astrocytes involves the control of the extracellular and neuronal concentrations of amino acid and biogenic amine neurotransmitters. Astrocytes take up extracellular glutamate (an excitatory amino acid transmitter) and metabolize it to glutamine which can recyle through the extracellular fluids back into neurons for conversion to glutamate. GABA is also taken up from the extracellular fluids and glutamate converted to glutamine for release and eventual capture by neurons. These arrangements have been termed “metabolic compartmentalization because the astrocytes are acting as a specific metabolic compartment for the surrounding neurons.
Following damage or trauma to the CNS structures the local astrocytes undergo a biochemical and morphological differentiation; this activation process is important in the protection of the surrounding CNS tissue. Activated astrocytes are termed “reactive astrocytes” and this process results in gliosis and the formation of a glial scar at the cite of injury (Ridet, Malhotra et al., 1997). In the activated state, reactive astrocytes produce oxidoreductase enzymes, proteases, protease inhibitors.
Astrocytes also appear to play a critical role in forming and maintaining synapses in the central nervous system. Studies have demonstrated that few synapses form in the absence of astrocytes and that synaptic number increases proportionately with increasing astrocyte number (Ullian, Sapperstein et al., 2001).
Oligodendrocytes
Oligodendrocytes are involved in the formation of myelin sheaths (Figure 5) in the central nervous system, and therefore act similar to the Schwann cells; only unlike their peripheral counterparts, they can ensheath multiple axons with myelin. A major component of the myelin sheath is a protein referred to a myelin basic protein. This protein is released in to the extracellular fluids and cerebrospinal fluid during the process of demyelination.
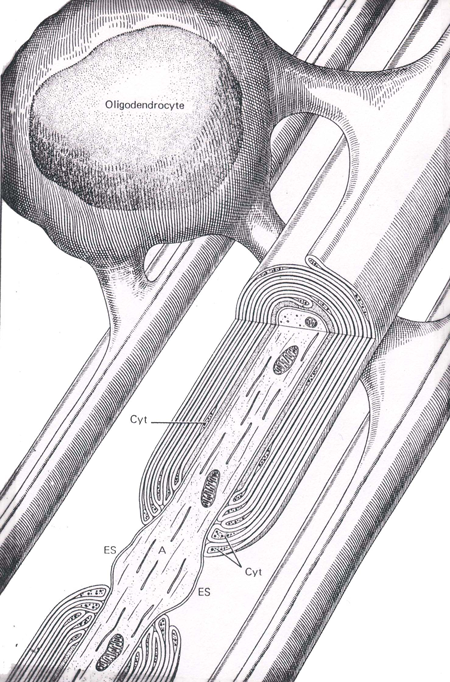
Figure 5 An illustration of an oligodendrocyte wrapping around several axons in the central nervous system (Abb.: A, axon; Cyt, glial cell cytoplasm; ES, extracellular space; IM, inner mesaxon; Taken from Junqueira, L.C., Carneiro, J., And Kelly, R.O., Basic Histology, Appleton and Lange, Norwalk, CT, 1989).
A principle concern with oligodendroglia is their susceptibility to disease (Raine, 1997). The consequence of their degeneration is demyelination. Genetic abnormalities in metabolism, infections and inflammation are all sources of oligodendrocyte demise. Autoimmune diseases such as multiple sclerosis feature an attack of oligodendrocyte myelin sheaths by activated T‑cells. A marker of the demyelination process seen in these diseases is the rise in myelin basic protein content of the cerebrospinal fluid (Davson, Welch et al., 1987).
Microglia
Microglia, which resemble tissue macrophages and provide the CNS with a first line of defense constitute 10-20% of the glial cells in the brain (Altman, 1994). It is generally agreed that microglia are bone marrow derived cells; they circulate as monocytes and gain access to the CNS by migrating through the endothelial linings of capillaries (Kreutzberg, 1996; Hickey and Kimura, 1988). As the brain macrophage system, microglia are capable of phagocytosis and of presenting antigens to lymphocytes. In addition, microglia produce a complex array of cytokines (Neumann and Wekerle, 1998).
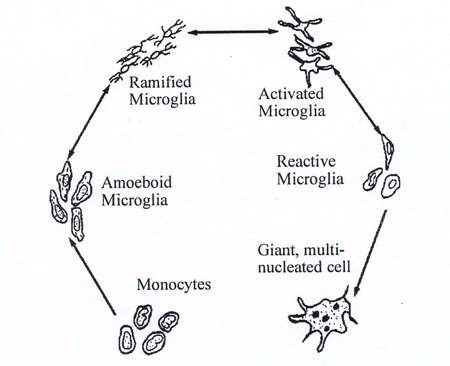
Figure 3 The life cycle of microglia in the central nervous system (modified from Davis, E.J., T.D. Foster, and W.E. Thomas (1994) Cellular forms and functions of brain microglia. Brain Res.Bull. 34:73-78)
Microglia have a complex life cycle in the brain (Figure 3). They arise as blood monocytes that migrate into the brain where they are trapped after development of the blood-brain barrier. Once in the brain they become amoeboid microglia with full macrophage capabilities. As the nervous system develops, these cells go into a quiescent stage, referred to as ramified microglia, with no macrophage capabilities. This resting condition can be converted into an active state termed an active microglia. These cells have partial macrophage capabilities, but can develop into reactive microglia with full macrophage capabilities (Davis, Foster et al., 1994).
In general, there are two major pools of microglia in the central nervous system: resting (or ramified) and activated which exist in an equilibrium. Activation occurs with tissue injury, usually by exposure to:
1) cytokines (IL-1, IL-2, IL-6, TGF-$1) released by other microglia or activated astrocytes,
2) major histocompatibility class I or class II molecules, or
3) increase neurotransmitters levels since microglia have receptors for many amino acid and biogenic amine neurotransmitters.
Activated microglia undergo proliferation, migration and immunologic differentiation. As professional phagocytes, activated microglia contain oxidative and cytotoxic enzymes and participate in destroying invading microorganisms, removing debris, as well as secreting healing factors to promote wound repair. All of these microglial activities are aimed at restoring original tissue homeostasis (Kreutzberg, 1996), however these events can go awry.
Microglia play a role in the pathogenesis numerous major diseases. In the AIDS‑dementia complex (Price, Brew et al., 1988), monocytes infected with the HIV virus may be the mechanism whereby the virus spreads to the CNS. Macrophages and giant, multinucleated cells derived from macrophages are a common feature of brain tissue taken from victims of the AIDS‑dementia complex (Navia, Cho et al., 1986). Inflammatory diseases of the brain involve dysregulation of cytokine release form glia, specifically microglia. Multiple sclerosis, a localized inflammatory process, is currently viewed as a produce of abnormal cytokine regulation. Demyelinating plaques in the CNS contain elevated amounts of proinflammatory cytokines, such as interleukin-1, interleukin-6 and tumor necrosis factor-a, secreted by surrounding leucocytes, macrophages and microglia (Brosnan, Cannella et al., 1995). During remission of the disease, remyelination occurs in the plaques and the anti-inflammatory cytokines, such as interleukin-4 and interleukin-10 increase in abundance as the proinflammatory cytokines decrease in content (Cannella and Raine, 1995). Abnormalities in microglial regulation are suspected in playing a role in the high levels of proinflammatory cytokine secretion during the active phase of the disease. Finally, microglia are also suspected of involvement in senile dementia of the Alzheimer’s type. This type of dementia can be modeled as a chronic inflammation of the brain brought on by a dysregulation of glial cytokines (Mrak, Sheng et al., 1995). Microglia are view as a source of interleukin-1, exacerbating this inflammatory process.
Ependymal cells
The lining of the cerebral ventricles and the central canal of the spinal cord is formed by a layer of simple cuboidal cells with a microvillous border, termed ependymal cells. (Figure 2). The outer surface of the ependymal cell is bathed in cerebrospinal fluid (CSF) while the inner border rests on a densely packed sheet of astrocytic end feet called the internal limiting membrane. Neither the ependymal cells or the glial membrane seem to represent any impediment to the exchange of material between the CSF and the brain. Ependymal cells, especially around the floor of the third ventricle, are involved in sampling the CSF and modulating neuronal activity in the hypothalamus to regulate body homeostasis.
Ependymal cells are vulnerable to injury at all ages (Sarnat, 1995). Injurious events include stretching during ventriculomegaly, infarction, hemorrhage, infections and inflammations, or exposures to cytotoxins. Since ependymal cells do not regenerate, the damaged area will become fibrotic with astrocytes. Perhaps the most devastating consequence occurs following infection of the ependymal cells with mumps or influenza virus. The ependymal cells expand in size while slothing off the ventricular walls, the resulting inflammatory process in the ventricular wall can close the narrow regions of the ventricular system such as in the cerebral aqueduct. This aqueductal stenosis can lead to hydrocephalus and severe built-up of pressure can have dire consequences for the brain.
Choroid plexus
During the development of the brain, the ependymal lining in portions of the lateral and fourth ventricles invaginate into the ventricular lumen. This invaination consists of a ball of blood vessels or vascular bud (Figure 2). Subsequently, the cuboidal ependymal cells form a lining surrounding the blood vessels of the vascular bud (a process similar to that of glomerular formation in the kidney). This structure, termed the choroid plexus, hangs into the ventricle. The lining cells of the choroid plexus have a microvillous ventricular border and are connected to each other through tight junctions. They are separated from the blood vessels by a well‑developed basal lamina. These modified ependymal cells play a secretory‑absorptive role and, in addition, act as an effective blood‑CSF barrier (Spector and Johanson, 1989).
Blood‑brain barrier
The world of neuronal function is very sensitive to ionic homeostasis in the surrounding tissue. Slight changes in chemical composition in the extracellular fluid could seriously impair neuronal activity. To protect the neuron a blood-brain barrier exist isolating the cell from the chemical medium contained in the extracellular fluid as well as the vascular system. The blood‑brain barrier (BBB) is a complex structure made up of the astrocytic cell processes, basal lamina and capillary endothelial cell (Goldstein and Betz, 1986). Just as the glial limiting membranes protect neurons from contact with harmful substances in the CSF, the BBB protects against contact with blood. The barrier does allow certain material to cross from blood to CNS tissue. Lipid soluble substances can diffuse across the barrier, in addition there are carrier molecules in the astrocyte membrane that transport other nutrient substances. The physical characteristics of the BBB are important in understanding the movement of pharmaceutical compounds into and out of the nervous system.
Cerebrospinal Fluid
The cerebrospinal fluid is a modified form of extracellular fluid that circulates in the subarachnoid spaces. It is an ultrafiltrate of plasma where most of the filtration is accomplished by the choroid plexus As such, its composition is very similar to that of extracellular fluid. The protein concentration varies from 10 mg (/100g) in ventricular fluid, to 15-20 mg protein (/100 g) in cisterna magna and 30-45 mg protein (/100 g) in lumbar subarachnoid space. Its electrolyte concentration is compared to that of plasma and intracellular fluid in the following table:
| Substance | Plasma | CSF | Intracellular |
| Sodium | 148 | 149 | 10 |
| Potassium | 4.3 | 2.9 | 159 |
| Magnesium | 2.02 | 1.74 | 40 |
| Calcium | 5.06 | 2.47 | <1 |
| Chloride | 106.0 | 130.0 | 3 |
| Bicarbonate | 25 | 22 | 7 |
Cerebrospinal fluid (CSF) is formed in two places: the choroid plexus and the central nervous system parenchyma. Approximately 70-80% of the CSF is formed in the ventricles by the choroid plexus. The remainder is in essence, extracellular fluid from the central nervous system that is extruded through the ventricular wall or through the pial surface to directly enter the subarachnoid spaces.
In a normal human, there is approximately 150 ml of CSF contained in the ventricles and subarchnoid spaces. Over the course of a day, we make approximately 500-600 ml of CSF or about 0.35 ml per minute. This rate of production has to be balanced by an equal rate of absorption of CSF through the arachnoid granulations of the superior sagittal sinus and the spinal granulations located distal to the dorsal root ganglion on the spinal nerve. Overproduction or under-absorption of CSF results in increasing intracranial pressure, a condition termed hydrocephalus. The expanding ventricles in hydrocephalus represent a medical emergency since they place increasing pressure on the central nervous system tissue which ultimately responds by reabsorption and cell loss.
